MS-SUPPORT Randomized Controlled Study
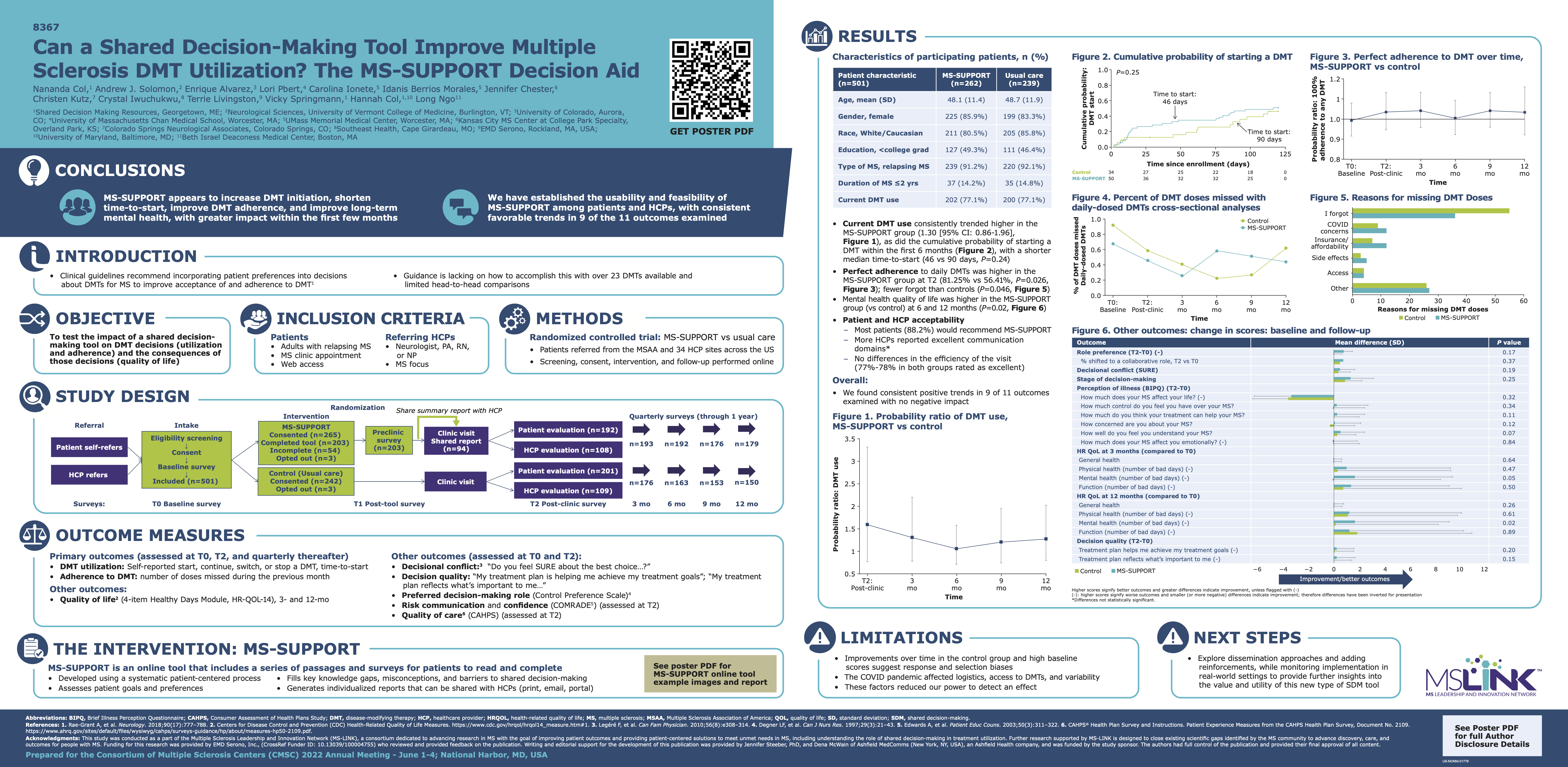
This study aims to validate the shared decision-making tool, MS-SUPPORT, in a sample of US adults with relapsing forms of MS and their HCPs. The study will assess: DMT utilization, patient-provider communication, DMT adherence, quality of decision making, quality of care, quality of life, decision conflict, and perception of illness
Partner: Col, Shared Decision Making Resources
Status: Complete





Outcomes Study
This study is a multi-center observational real-world platform which aims to integrate data from multiple sources to facilitate patient and provider engagement, shared decision making, and participation in high-priority research questions, as well as identify a core set of patient reported outcomes (PROs) that correlates with disease progression and activity
Partner: Tornatore, MedStar Georgetown; English, MS Center of Atlanta
Status: Ongoing








Truven Treatment in MS Analysis
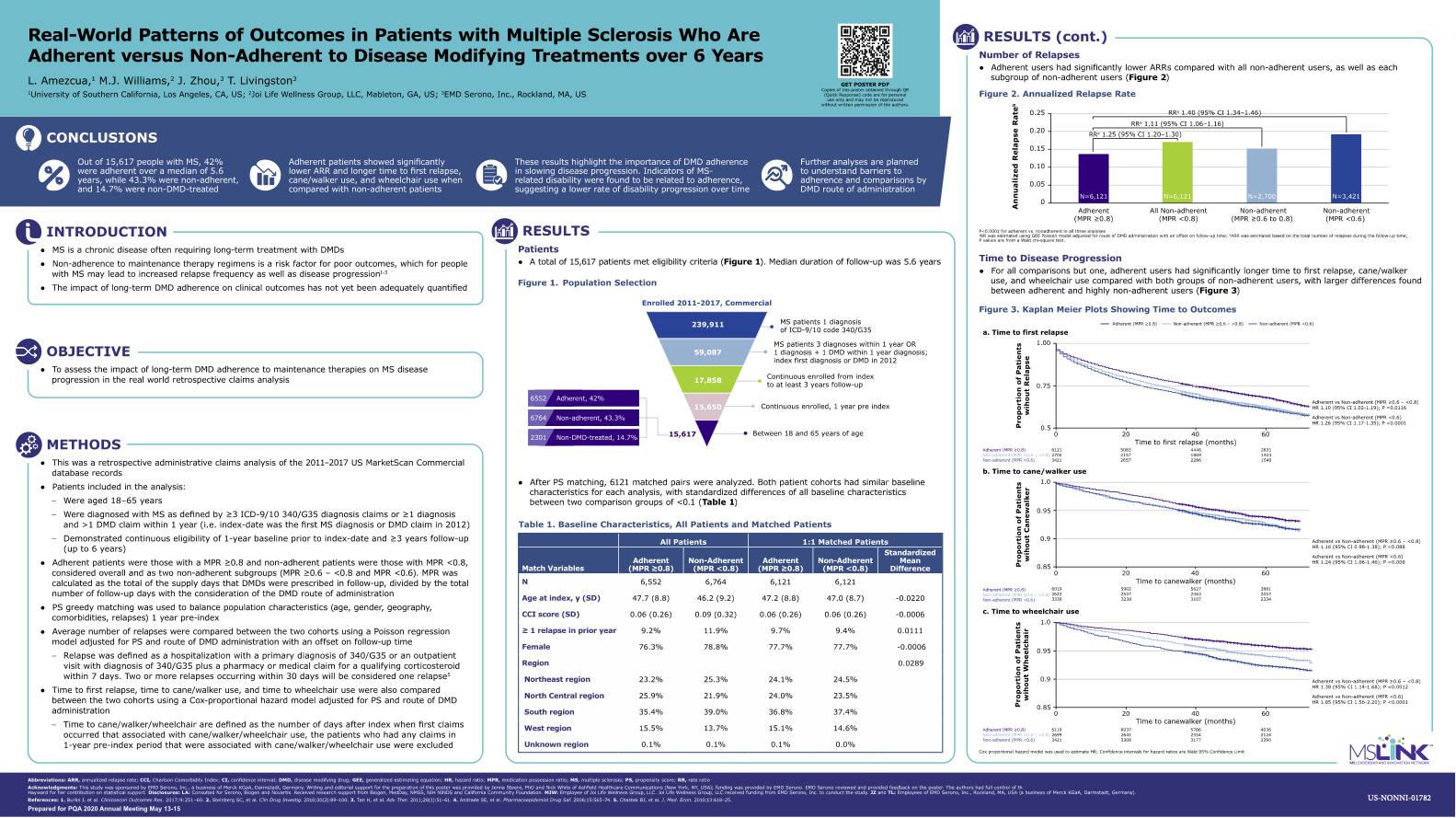
Evaluate disease progression and health resource utilization (HRU) in people with MS (PwMS) treated with a disease modifying treatment (DMT): adherent users versus non-adherent users over 6 years
Partner: Amezcua, USC; Williams, Joi Wellness
Status: Complete