Outcomes Study
This study is a multi-center observational real-world platform which aims to integrate data from multiple sources to facilitate patient and provider engagement, shared decision making, and participation in high-priority research questions, as well as identify a core set of patient reported outcomes (PROs) that correlates with disease progression and activity
Partner: Tornatore, MedStar Georgetown; English, MS Center of Atlanta
Status: Ongoing








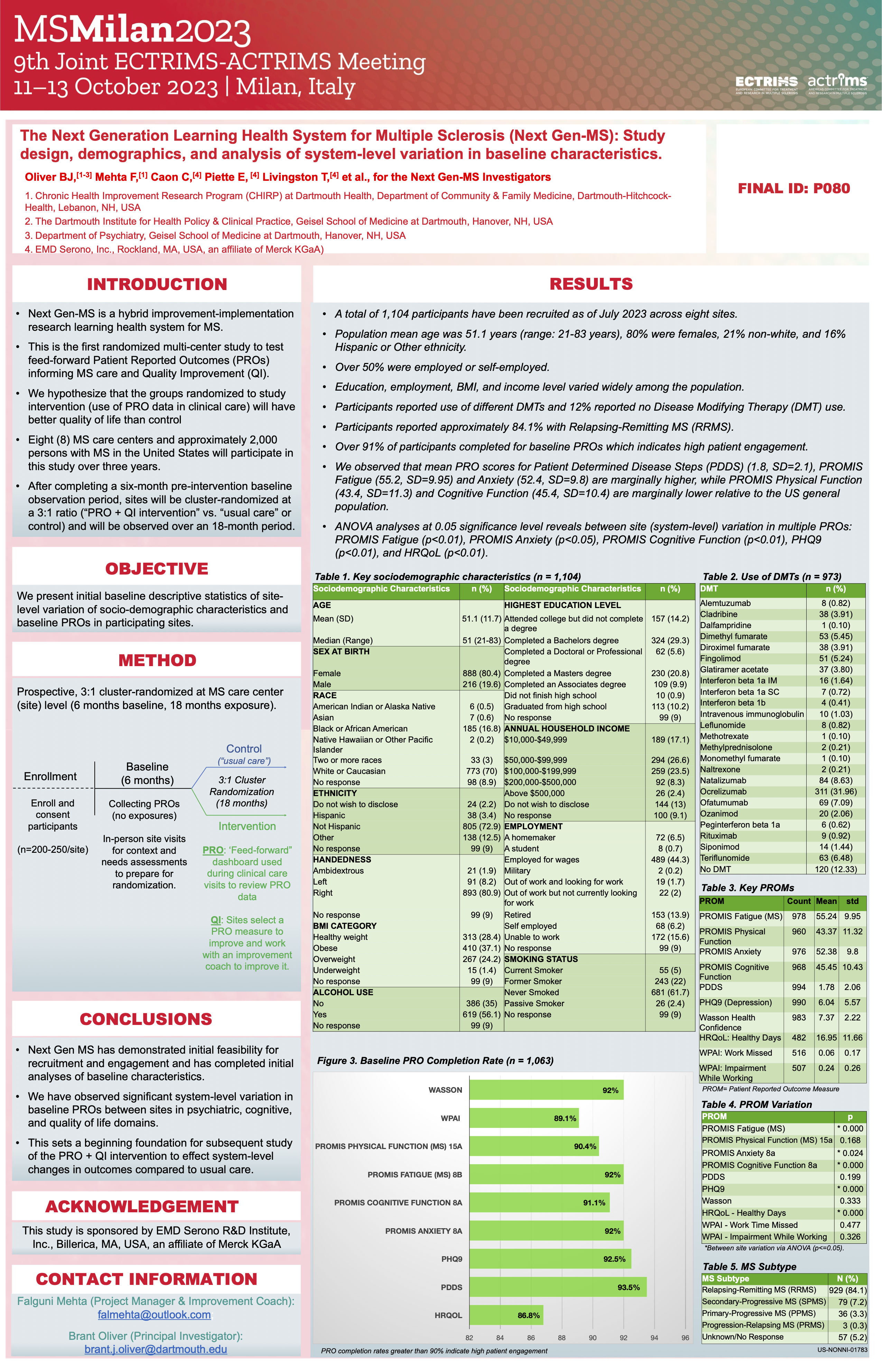
Next Gen MS
This study will use PRO data collected in the MS-LINK Outcomes study in a feed-forward method to evaluate if PRO data can be practically utilized within real healthcare systems to improve quality of life or delay disability progression in adults living with MS
Partner: Oliver, Dartmouth
Status: Ongoing





DISCOMS Extension
This study aims to provide data on the durability of disease inactivity, and potential risks, after discontinuing DMT(s) in MS
Partner: Cutter, University of Alabama; Corboy, University of Colorado
Status: Complete



